would you like to know all about distilled spirits? The world of liqueurs is a wide range that has existed for thousands of years, and since then many liqueurs have been made by distilling alcohol. Distillation can result in an essentially complete separation composed of almost pure components, or it can be a partial separation that increases the concentration of selected components in the mixture, join us to discover more about this process.
En esta nota, encontraras lo siguiente
what is alcohol distillation?
Alcohol distillation is a process that consists of heating a liquid until its most volatile components pass into the vapor phase and then cooling the vapor to recover these components in liquid form by condensation.
The main objective of distillation is to separate a mixture of several components by taking advantage of their different volatilities, or to separate the volatile materials from the non-volatile ones. If the difference in volatility and boiling point between the two components is large, complete separation can be easily accomplished in a single distillation.
So we can understand for example that seawater containing 4% dissolved solids can be easily purified by evaporating the water, and then condensing the vapor to collect the product, which turns out to be distilled water.
what is the process of separating water and alcohol?
The process of separating water and alcohol can be carried out in different ways, the most commonly used method is to heat the mixed liquid, since alcohol has a lower boiling temperature than water, so it will evaporate more quickly. 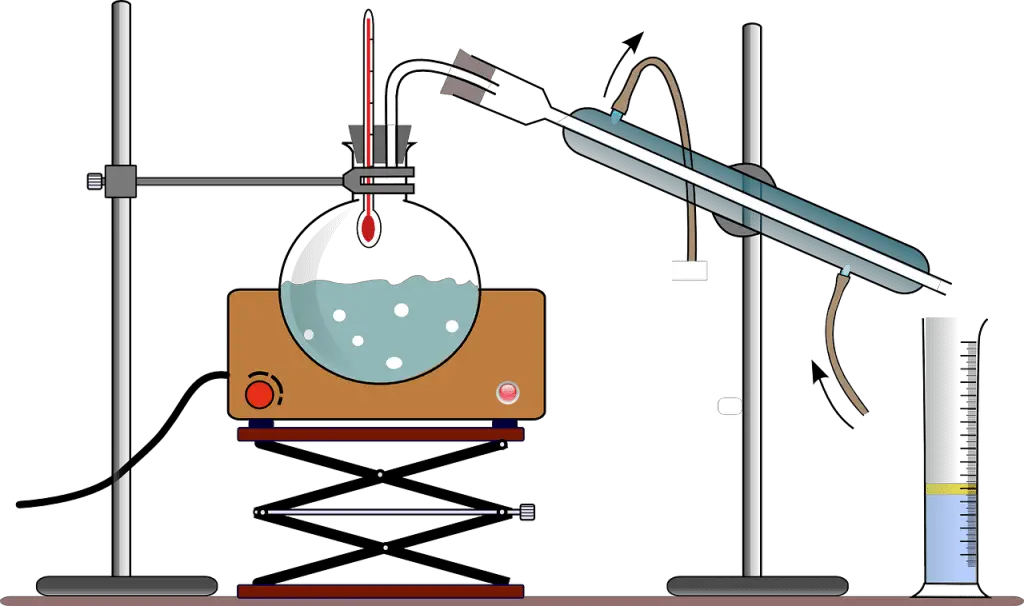 To separate a mixture of alcohol and water, the distillation variant known as fractional distillation is often used, which is used when the components of the solution have close boiling points, at most about 25 degrees Celsius.
To separate a mixture of alcohol and water, the distillation variant known as fractional distillation is often used, which is used when the components of the solution have close boiling points, at most about 25 degrees Celsius.
This fractional distillation includes to the distillation apparatus the component or part known as fractionating column. Ethyl alcohol boils at a temperature of 78.4°C, while water has a boiling point close to 100°C, only 21.6°C difference is determined making a fractionating column necessary.
At about 80°C the alcohol evaporates, while water tends to remain liquid, if we manage to maintain the temperature at this point the gaseous azeotropic mixture will seek to have the same concentration as in the liquid state. To elaborate a fractional alcohol distillation equipment it is necessary to keep in mind the following:
- A round bottom flask
- Fractionation column
- A liquid condenser
- Flat bottom flask or beaker
- Burner
Once you have obtained the instruments you will have to:
- Pour the ethanol and water mixture into the round bottom flask.
- Assemble the fractional distillation apparatus by attaching the column to the round bottom flask.
- Attach the condenser to the fractionating column and place the beaker to capture the distillation underneath.
- Place the burner under the round bottom flask and heat the mixture to the boiling point of ethanol. Here it is important to keep the temperature around 80°C.
- You will observe in the process, how small drops start to precipitate in the condenser tube and from there into the beaker, giving rise to a more concentrated ethyl alcohol mixture than the initial one.
how to Distill Alcohol?
Distilling alcohol is a process that consists of heating a liquid until its most volatile components pass to the vapor phase and then cooling this vapor to recover the components in liquid form by condensation. In order to carry out the alcohol distillation process you must perform the following steps:
- Place the mixture of the liquids in the distillation flask.
- Place a stopper in the mouth of the flask and a thermometer to control the temperature.
- To heat the mixture, this will allow to enter first in boiling the liquid of smaller boiling point.
- Once boiling, the vapor will rise through the cooling tube.
- Let water pass through the cooling tube, this will allow to reduce the temperature of the vapor by condensing it.
- Collect the distillate in a flask.
what are the Types of Alcohol Distillation?
The alcohol distillation process can be carried out in different ways, so there is not only one type of distillation, we can find diversities that will allow us to obtain the final product in less or more time. 
Simple distillation
This is the simplest type of alcohol distillation and consists of boiling the mixture until the elements are separated.
Fractional distillation
It is carried out by means of a fractionation column in which different plates successively produce evaporation and condensation, guaranteeing greater purity in the result.
Vacuum distillation
A distillation process in which the vacuum pressure generated is used and the process is catalyzed to reduce the boiling point of the ingredients by half.
Azeotropic distillation
This is used to break down an azeotrope, i.e. a mixture whose substances behave as one, even sharing a boiling point.
Steam distillation
In this type of distillation, the volatile and non-volatile components of a mixture are separated by direct injection of water vapor.
Dry distillation
This consists of heating solid materials without the presence of liquid solvents, obtaining gases and then condensing them in another container.
Enhanced distillation
It is also known as alternate or reactive distillation, and is used for mixtures that are difficult to separate or of the same boiling point.